Rybelsus (Semaglutide)











Dosages
Rybelsus 3 mg
| Quantity | Price per pill | Total price | |
|---|---|---|---|
| 10 | $25.00 | $250.00 | |
| 20 | $21.00 | $420.00 | |
| 30 | $18.33 | $550.00 |
Rybelsus 7 mg
| Quantity | Price per pill | Total price | |
|---|---|---|---|
| 10 | $33.00 | $330.00 | |
| 20 | $28.00 | $560.00 | |
| 30 | $26.67 | $800.00 |
Rybelsus 14 mg
| Quantity | Price per pill | Total price | |
|---|---|---|---|
| 10 | $36.00 | $360.00 | |
| 20 | $31.00 | $620.00 | |
| 30 | $29.33 | $880.00 |
Payment & Shipping
Your order is securely packed and usually ships within 24 hours. Here's what a typical package looks like.
It is about the size of a regular letter (9.4x4.3x0.3 inches) and shows no details about what is inside.



| Shipping Method | Estimated delivery |
|---|---|
| Express Free for orders over $300.00 | Estimated delivery to the U.S.: 4-7 days |
| Standard Free for orders over $200.00 | Estimated delivery to the U.S.: 14-21 days |









Discount Coupons
- Independence Day - July 4, 2026 10% JULY410
- Labor Day - September 7, 2026 7% LABOR07
- Thanksgiving - November 26, 2026 9% THANKS09
Brand Names
| Manufacturer | Brand Names |
|---|---|
| Novo Nordisc India Pvt. Ltd | - |
FAQ
Description
Rybelsus is a brand name for an oral medication used to treat type 2 diabetes. Rybelsus is unique because it's the first oral GLP-1 receptor agonist available for diabetes management. GLP-1 receptor agonists help lower blood sugar levels by stimulating insulin release and reducing the amount of glucose produced by the liver. Rybelsus is typically prescribed as part of a comprehensive treatment plan that includes diet and exercise. As with any medication, individuals need to follow their healthcare provider's guidance and monitor their blood sugar levels regularly while taking Rybelsus.
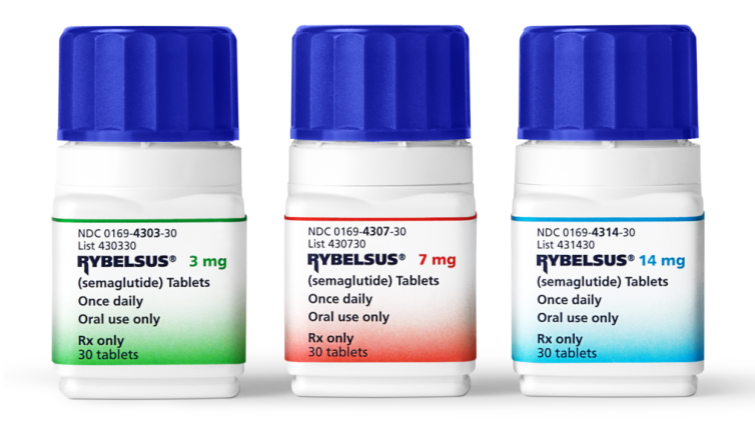
What Are the Ingredients of Rybelsus?
The active ingredient in Rybelsus is semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist. Semaglutide works by mimicking the action of the natural hormone GLP-1, which regulates blood sugar levels. GLP-1 receptor agonists like semaglutide stimulate insulin secretion, reduce the amount of glucose produced by the liver, and slow down glucose absorption from the digestive system.
Aside from the active ingredient, Rybelsus tablets also contain various inactive ingredients, which are used to form the tablet and improve its stability, appearance, and taste. These inactive ingredients can include fillers, binders, disintegrants, and other excipients. The specific formulation of these inactive ingredients can vary among different pharmaceutical manufacturers, so you need to check the product's package insert or consult their healthcare provider for detailed information about the specific composition of the Rybelsus tablets they are using.
What Are the Forms of the Rybelsus?
Rybelsus is available in the form of 3 mg, 7mg, or 14 mg oral tablets. These tablets are taken orally, and they contain the active ingredient semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist. The tablet form distinguishes Rybelsus from other GLP-1 receptor agonists, as it is the first oral medication in this class.
An oral tablet's convenience can appeal to individuals who prefer this form of administration over injectable options.
NDC Code(s): 0169-4303-30, 0169-4307-30, 0169-4314-30.
Uses of Rybelsus
Rybelsus is primarily used for the treatment of type 2 diabetes in adults. As a glucagon-like peptide-1 (GLP-1) receptor agonist, it helps regulate blood sugar levels by stimulating insulin release, reducing glucose production by the liver, and slowing down glucose absorption from the digestive system.
In addition to its role in managing blood sugar, Rybelsus may also have other potential benefits. Some studies suggest that GLP-1 receptor agonists, like semaglutide in Rybelsus, may contribute to weight loss, as they can promote feelings of fullness and reduce appetite.
The medication is typically prescribed as part of a comprehensive treatment plan, including diet, exercise, and other lifestyle modifications.
Dosage
The dosage of Rybelsus can vary based on individual factors such as the patient's medical condition, response to treatment, and other medications they may be taking. Generally, Rybelsus is initiated at a lower dose and then gradually increased to achieve optimal blood sugar control.
For specific dosage instructions, it's essential to follow the healthcare provider's recommendations outlined in the prescription. Typically, Rybelsus is taken once daily, with or without food. The tablets should be swallowed whole with a glass of water and not crushed or split.
It's crucial to attend regular follow-up appointments with a healthcare provider to monitor their response to Rybelsus and make necessary adjustments to the dosage or treatment plan. Dosage adjustments may be made based on weight changes, other medications, and overall glycemic control.
Important Safety Information
Rybelsus may lower blood sugar levels, and hypoglycemia (low blood sugar) can occur. It's essential to be aware of the signs and symptoms of hypoglycemia, such as sweating, shakiness, dizziness, and confusion. Individuals should be educated on how to manage low blood sugar, including the use of glucose tablets or other sources of quick-acting carbohydrates.
There have been reports of pancreas inflammation (pancreatitis) in individuals using GLP-1 receptor agonists. If signs and symptoms of pancreatitis, such as severe abdominal pain, occur, individuals should seek medical attention promptly.
GLP-1 receptor agonists have been associated with an increased risk of thyroid C-cell tumors in animal studies. While the relevance of this finding to humans is unclear, individuals with a history of medullary thyroid carcinoma or a family history of multiple endocrine neoplasia syndrome type 2 should avoid Rybelsus.
Common side effects of Rybelsus include nausea, vomiting, and diarrhea, particularly during the initiation of treatment. These side effects may decrease over time.
Rybelsus should be used with caution in individuals with renal impairment, and kidney function should be monitored regularly.
You must inform your healthcare provider of their medical history, including any existing health conditions or medications they are taking, before starting Rybelsus. This information will help healthcare providers make informed decisions about the appropriateness of Rybelsus for each individual. As with any medication, the benefits and risks should be discussed thoroughly with a healthcare professional.
Contraindication
Rybelsus is contraindicated in individuals with a history of a severe hypersensitivity reaction to semaglutide or any of the other components in the medication. Hypersensitivity reactions can manifest as severe allergic reactions and may include symptoms such as swelling of the face, lips, tongue, or throat, difficulty breathing, or severe itching.
Additionally, Rybelsus is not recommended for use in individuals with a personal or family history of medullary thyroid carcinoma or patients with multiple endocrine neoplasia syndrome type 2. This is due to the potential risk of thyroid C-cell tumors associated with GLP-1 receptor agonists.
Before starting Rybelsus or any medication, individuals should provide their healthcare provider with a comprehensive medical history, including information about allergies, existing health conditions, and any medications they are currently taking. This helps healthcare professionals assess the suitability of Rybelsus for each individual and make informed decisions about their diabetes management plan. If there are concerns or questions about contraindications, individuals should discuss them with their healthcare provider.
Interactions
Rybelsus may interact with other medications, and individuals need to inform their healthcare provider about all the medications, supplements, and herbal products they are taking before starting Rybelsus. Some potential interactions include:
- Insulin or Insulin-Secretagogues: Hypoglycemia (low blood sugar) may increase when Rybelsus is combined with insulin or insulin-secretagogues (medications that stimulate insulin release).
- Oral Medications: Rybelsus may affect the absorption of certain oral medications. It's important to discuss with a healthcare provider if other oral medications are being taken and adjustments to the timing or dosage may be necessary.
- Warfarin: There have been reports of an increased international normalized ratio (INR) and bleeding in patients taking warfarin and semaglutide (the active ingredient in Rybelsus). Close monitoring of INR is recommended in individuals taking warfarin with Rybelsus.
- CYP Enzyme Inhibitors and Inducers: Rybelsus is metabolized by specific enzymes in the liver, and medications that inhibit or induce these enzymes may affect the concentration of Rybelsus in the body. It's essential to inform the healthcare provider about any medications that fall into these categories.
- Gastrointestinal Medications: Certain medications used to treat gastrointestinal disorders may affect the absorption of Rybelsus. It's important to discuss the use of these medications with a healthcare provider.
Individuals must communicate openly and transparently with their healthcare providers about their medications. This helps ensure the safe and effective use of Rybelsus and minimizes the risk of potential interactions. Adjustments to medication regimens may be necessary based on individual health factors and treatment goals.
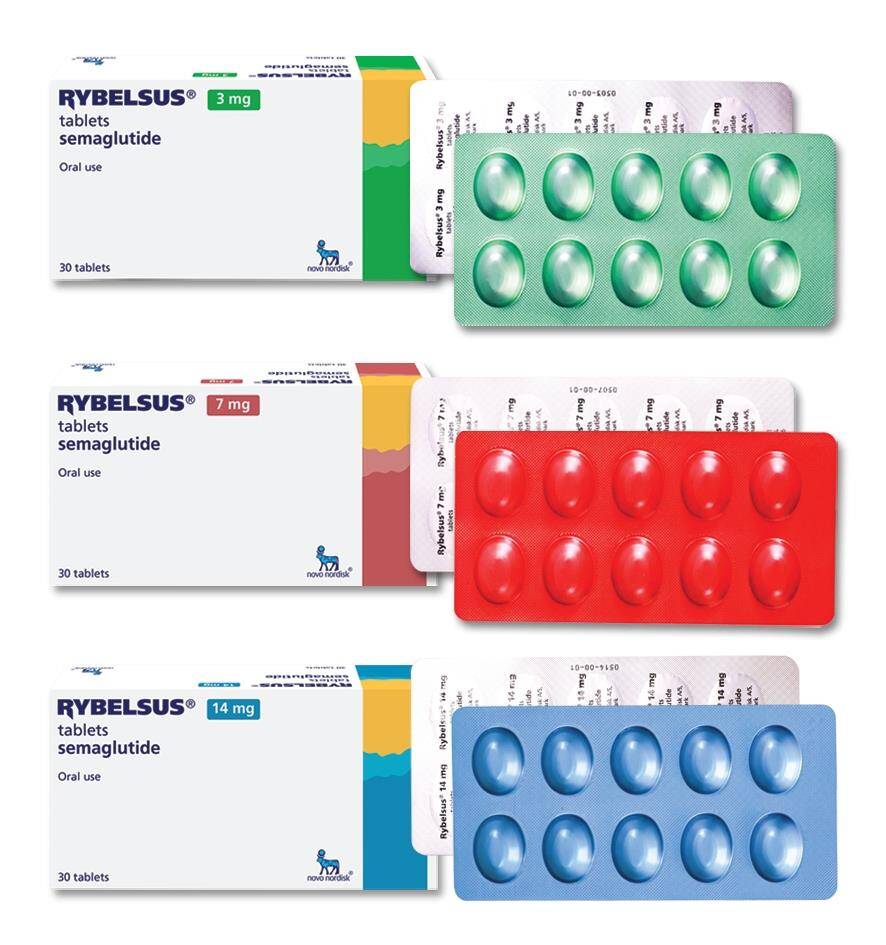
Side Effects
Like any medication, Rybelsus may cause side effects. Common side effects include gastrointestinal symptoms such as nausea, vomiting, and diarrhea, particularly during the initiation of treatment. These side effects often diminish over time as the body adjusts to the medication.
Other potential side effects of Rybelsus may include:
- Hypoglycemia: As Rybelsus works to lower blood sugar levels, there is a risk of hypoglycemia (low blood sugar). Symptoms may include sweating, shakiness, dizziness, and confusion.
- Pancreatitis: There have been reports of inflammation of the pancreas (pancreatitis) in individuals using GLP-1 receptor agonists, although the incidence is rare.
- In animal studies, thyroid C-cell tumors: GLP-1 receptor agonists have been associated with an increased risk of thyroid C-cell tumors. The relevance of this finding to humans is unclear.
- Renal Impairment: Rybelsus should be used cautiously in individuals with renal impairment, and kidney function should be monitored regularly.
You must promptly report any unusual or severe side effects to your healthcare provider. In some cases, the benefits of Rybelsus in managing diabetes may outweigh the potential risks. Healthcare providers can guide managing side effects and adjust the treatment plan as needed. If there are concerns about side effects, discussing them with a healthcare professional is advisable.
Overdose
In case of an overdose with Rybelsus, individuals should seek immediate medical attention or contact their local poison control center. Overdose symptoms may include severe nausea, vomiting, and signs of hypoglycemia (low blood sugar), such as confusion, shakiness, and sweating.
Take Rybelsus precisely as their healthcare provider prescribes and not exceed the recommended dosage. If a dose is missed, individuals should follow the healthcare provider's instructions on what to do.
Treatment of Rybelsus overdose may involve supportive measures to address symptoms, such as intravenous glucose for hypoglycemia. The specific treatment will depend on the severity of the overdose and the individual's overall health.
As with any medication, it's crucial to keep Rybelsus out of reach of children and pets to prevent accidental ingestion. If an overdose is suspected, immediate medical attention is essential to ensure appropriate care and minimize potential complications.
How to Store
Rybelsus should be stored according to the specific instructions provided by the manufacturer and healthcare provider. In general:
Rybelsus tablets should be stored at room temperature, typically between 68°F to 77°F (20°C to 25°C). It's important to avoid extreme temperatures and store the medication away from heat and moisture.
Keep Rybelsus in its original blister packaging until ready to use. The blister packaging helps protect the tablets from environmental factors.
Store Rybelsus out of reach of children and pets to prevent accidental ingestion.
Rybelsus tablets should not be transferred to another container. Please keep them in the original blister packaging until ready to take a dose.
Before taking Rybelsus, individuals should check the expiration date on the packaging. Expired medication should not be used.
It's essential to follow any additional storage instructions the healthcare provider or pharmacist provides. Proper storage helps maintain the effectiveness and safety of the medication.
Drugs Similar to Rybelsus
Rybelsus belongs to the class of medications known as glucagon-like peptide-1 (GLP-1) receptor agonists. Several other GLP-1 receptor agonists are available, and they may be considered similar in terms of their mechanism of action and use in managing type 2 diabetes. Some examples of GLP-1 receptor agonists include:
- Victoza (liraglutide): Administered by injection, Victoza is another GLP-1 receptor agonist used to improve blood sugar control in adults with type 2 diabetes. It is given once daily.
- Trulicity (dulaglutide): Trulicity is a once-weekly injectable GLP-1 receptor agonist used to treat type 2 diabetes.
- Bydureon (exenatide extended-release): Bydureon is an extended-release formulation of exenatide, a GLP-1 receptor agonist. It is typically administered once weekly.
- Ozempic (semaglutide): Similar to Rybelsus, Ozempic is a GLP-1 receptor agonist, but it is administered by subcutaneous injection. It improves blood sugar control in adults with type 2 diabetes.
These medications, like Rybelsus, work to lower blood sugar levels by stimulating insulin release, reducing glucose production by the liver, and slowing down glucose absorption from the digestive system. However, the choice of medication may depend on individual factors such as patient preferences, response to treatment, and the mode of administration (oral or injectable). Healthcare providers will consider these factors when prescribing medication for diabetes management.
FAQ
How does Rybelsus differ from other diabetes medications?
Rybelsus is unique as it is the first oral medication in the class of GLP-1 receptor agonists, providing an alternative to injectable options for diabetes management.
Are there any dietary restrictions while taking Rybelsus?
While there are no specific dietary restrictions, individuals should follow a healthy diet as part of their overall diabetes management. It's important to discuss dietary considerations with a healthcare provider.
What should I do if I miss a dose of Rybelsus?
If a dose of Rybelsus is missed, individuals should take it as soon as they remember. They should skip the missed dose and resume their regular dosing schedule if it is almost time for the next dose.
How quickly does Rybelsus start working to lower blood sugar levels?
Rybelsus may start working to lower blood sugar levels within a few days to weeks, but individual responses may vary. It's essential to monitor blood sugar levels as directed by a healthcare provider.
Can Rybelsus be used during pregnancy or breastfeeding?
The safety of Rybelsus during pregnancy and breastfeeding has not been established, and it should be used with caution in these situations. Pregnant or breastfeeding individuals should consult their healthcare provider.












