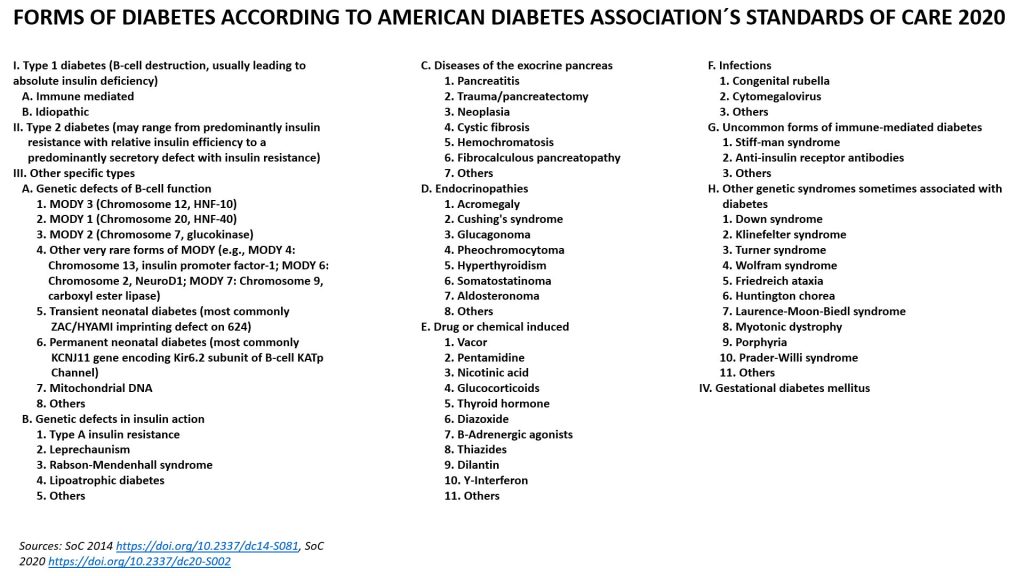
The picture at the top of this side shows the most common forms of diabetes with other words, here is a more comprehensive version including all we know today, from the American Diabetes Associations Standards of Care 2020 and 2014 (https://doi.org/10.2337/dc14-S081, SoC 2020 https://doi.org/10.2337/dc20-S002):
AUTOIMMUNE DIABETES
Type 1 diabetes and LADA (Latent Autoimmune Diabetes in Adults, slower onset of autoimmune diabetes in people above ~35 years) are autoimmune diseases which means the immune system mistakenly attacks the body´s healthy tissues (1, 2). The only thing being attacked are the beta cells in the islets of Langerhans in the pancreas, and this leads to absolute insulin deficiency. You can´t survive without insulin. Example of other autoimmune diseases are rheumatoid arthritis, multiple sclerosis and Guillain-Barré syndrome. The etiology of autoimmune diabetes is unknown (Nov 2018), we know that risk genes is a must but it´s believed ~30-50% of the risk is due to heredity (3) This risk is mainly linked to HLA region on chromosome 6, with the greatest association with HLA class 2 haplotypes DR3-DQ2 and DR4-DQ8. These genes are common in the Nordics, about 20-25% of the population have any of these. Biomarkers for diagnose are, except from risk genes, low C-peptide (except from LADA, where C-peptide at diagnose can be similar as for a person with type 2 diabetes but decrease faster than with type 2) and autoantibodies. Autoantibodies can be detected years before diagnose and symptoms for a disease. Little is known of the progression from when autoimmunity appears to diagnose, except from that more autoantibodies in general means increased risk for autoimmune diabetes as well as time from autoimmunity to diagnose is shorter (4). It also seems that the progression is more aggressive in children than adults. The longest known time for a person with detected autoantibodies to diagnose is from Finland. A woman had autoantibodies at the age of 11 and was diagnosed when she was 32 years (5). There are five known autoantibodies today (6, 7) even though the value of the latest finding, Tetraspanin 7, is unknown.
Researchers see a quite strong link with enteroviruses, a group with ~100 viruses, where coxsackie b-virus (CBV) is a small subgroup with 6 different viruses. If CBV is involved we don´t know but many studies have seen a correlation. Trial in humans will start in Finland 2018 with a CBV vaccine. If it works the idea is not a cure but hopefully possible as prevention (8). Spring 2018 a group of scientists from Cincinnati Children’s Hospital Medical Center with a computational method (9) demonstrated a link with seven autoimmune diseases and Epstein-Barr virus (EBV). EBV have been a candidate virus even though stronger correlation has been seen with other viruses. The hypothesis today is that environmental factors are involved in the process as ”triggers”, if so and which is unknown. Autoimmune diabetes is a heterogeneous disease, it might be different ”triggers” depending on age at diagnose, which autoantibodies that occur and perhaps other factors. Autoimmune diabetes is chronic and can today not be prevented, even if biomarkers are detected. It can happen at any age. Treatment is insulin with MDI (Multiple Daily Injections, syringes) or an insulin pump.
References:
- https://www.nature.com/articles/nrdp201716
- https://doi.org/10.1007/s00125-015-3789-z
- http://care.diabetesjournals.org/content/38/10/1964
- https://www.ncbi.nlm.nih.gov/pubmed/28597949
- http://care.diabetesjournals.org/content/33/6/1206.long
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4407751/
- https://www.ncbi.nlm.nih.gov/pubmed/27221092
- https://www.uta.fi/en/news/story/preventive-vaccine-type-1-diabetes-be-studied-humans-first-time
- https://www.nature.com/articles/s41588-018-0102-3
TYPE 2 DIABETES
The etiology behind type 2 diabetes is genetics and lifestyle factors. The heredity is stronger for type 2 diabetes than for type 1 diabetes (1, 2). It is established ~90% are obese, but that means not all are (3). In UK 12.4% of people aged 18 years and over with obesity have diagnosed diabetes, five times that of people with a healthy weight but still not all obese people get type 2 diabetes, even if some may have prediabetes (4). Many become resistant to insulin and some have metabolic syndrome (5). One exciting research area last years is epigenetics, of which a lot is still unknown (6, 7). The ”western lifestyle” is of course not alone to blame for the increased prevalence of type 2 diabetes, but the envorinment we live in definitely doesn´t help people to make the best choices. We must together work to change the stigma, it doesn´t help people who are obese or have type 2 diabetes if the society is telling them ”blame your self”. To be considered as well, type 1 diabetes have higher mortality and is a more severe disease, but that doesn’t mean type 2 diabetes is harmless. It´s not black and white.
Last year’s some researchers have argued that type 2 diabetes is a heterogeneous disease, even more than 1 diabetes, and a subcategorization have been proposed (8, 9). This is still far from accepted. The idea is of course precision medicine, to give the best possible treatment and outcome. On the other side, some researchers mean that people are heterogeneous indeed, but not type 2 diabetes: it´s still about too much fat. Of several promising trials, most interesting so far is Roy Taylor et al research, performed in UK. They published a great result in The Lancet December 2017 (10) where the participants (in total 306, intervention 149) had a maximum duration of 6 years and were not treated with insulin. Intervention was; “The intervention comprised withdrawal of antidiabetic and antihypertensive drugs, total diet replacement (825–853 kcal/day formula diet for 3–5 months), stepped food reintroduction (2–8 weeks), and structured support for long-term weight loss maintenance. Primary outcome: “Co-primary outcomes were weight loss of 15 kg or more, and remission of diabetes, defined as glycated haemoglobin (HbA1c) of less than 6,5% (<48 mmol/mol) after at least 2 months off all antidiabetic medications, from baseline to 12 months.” Incredible result, diabetes remission was achieved in 68 participants (46%) who got off antidiabetic drugs. In a later, deeper analysis, they looked what happened, and found that remission requires decrease in liver and pancreas fat but is dependent upon capacity for beta cell recovery (11). In the follow up after two years, 53/149 (35,6%) of those commencing the intervention and 5/149 (3,4%) in the control group had remission, and 11,4% (17/149) of intervention and 2% (3/149) of the control group had weight loss ≥15kg. Of those maintaining ≥10kg weight loss (45/272), 64% (29/45) achieved remission (12). NOTE: This is a very restrictive diet and should not under any circumstances being implemented without consulting your endo. This is experimental and even though promising not yet widely accepted. Also please remember that study participants did not yet use insulin.
Even though lifestyle is not only to blame and genetics have impact, type 2 diabetes is highly preventable. Studies are showing different results, the Diabetes Prevention Program showed that intensive lifestyle intervention could reduce the incidence of type 2 diabetes by 58% over 3 years (13). Nevertheless, more actions are needed to help people take more rational and better decisions. Current treatment for type 2 diabetes is lifestyle changes, medicines and after some years with the disease most people need insulin (14). So still, despite promising results in trials, type 2 diabetes is a chronic disease without a cure.
References:
- https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/type-2-diabetes
- https://professional.diabetes.org/sites/professional.diabetes.org/files/media/type_2_1.pdf
- http://sciencenordic.com/slim-and-healthy-people-also-get-type-2-diabetes
- https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/338934/Adult_obesity_and_type_2_diabetes_.pdf
- https://www.nhs.uk/conditions/metabolic-syndrome/
- http://www.ludc.med.lu.se/news-archive/epigenetic-changes-could-explain-type-2-diabetes/
- http://onlinelibrary.wiley.com/doi/10.1111/jdi.12724/pdf
- http://www.thelancet.com/journals/landia/article/PIIS2213-8587(18)30051-2/fulltext?elsca1=tlpr
- https://www.medscape.com/viewarticle/893305
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)33102-1/fulltext
- https://www.cell.com/cell-metabolism/fulltext/S1550-4131(18)30446-7#secsectitle0020
- https://www.directclinicaltrial.org.uk/Pubfiles/Final%20accepted%20draft,%20prior%20to%20editing%20and%20corrections.pdf
- http://care.diabetesjournals.org/content/41/Supplement_1/S51
- https://emedicine.medscape.com/article/117853-treatment
MONOGENIC DIABETES
Monogenic diabetes are several forms of diabetes that results from a mutation in a single gene. Most common form of monogenic diabetes is MODY (Maturity Onset Diabetes of the Young), which account for 1-6% of pediatric diabetes. So far six different forms of MODY have been identified, and these account for 87% of MODY in UK (1). Some studies suggest there might be fourteen forms but yet to be confirmed. This means there are some genes to identify though. Characteristic is that most people that are diagnosed are below 25 years, one of the parents have the disease (strong heredity) and most people don´t need insulin. Most people remain production of insulin, at different degrees, but have limited secretion. Treatment is normally diet or tablets. MODY is sometimes confused with type 1 or 2 diabetes, which would be avoided with a genetic test. Correct diagnose with the right treatment if of course important for best possible outcome.
Other monogenic forms of diabetes is neonatal diabetes (a rare form with an incidence of ~1/100 000 births), a rare form with insulin resistance due to a genetic defect and others (1, 2, 3).
References:
- https://www.diabetesgenes.org/what-is-mody/
- https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/monogenic-neonatal-mellitus-mody
- https://ghr.nlm.nih.gov/condition/maternally-inherited-diabetes-and-deafness#genes
GESTATIONAL DIABETES
At pregnancy about 3-14% of women become resistant to insulin (1). Some can´t compensate with increased insulin production and develop gestational diabetes. Most people manage through lifestyle changes but some need insulin. Women who have had gestational diabetes are at risk to develop type 2 diabetes (2), studies showing different future risks though.
References:
- https://emedicine.medscape.com/article/127547-overview#a2
- https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes/gestational
SECONDARY DIABETES
A lot of different processes that affect the pancreas can cause a reduction in beta cell mass, and diabetes. The most common cause of secondary diabetes, or type 3c diabetes, is recurrent acute or chronic pancreatitis, inflamed pancreas. But also pancreatic cancer, haemochromatosis, cystic fibrosis, trauma, infection are known reasons that might induce diabetes as well (1, 2, 3). The most obvious form of secondary diabetes is that following partial or complete surgical resection of the pancreas (4).
In 2017 British researchers published a study in Diabetes Care (5) which got a lot of global attention. Headlines from many was; “a new form of diabetes, type 3c diabetes, has been discovered”. The form is not new and neither is the name 3c as you see in the links above. However, the researchers found in an analysis of 30 000 British people newly diagnosed with diabetes that 559 people had type 3c diabetes. Of these, ~90% were misdiagnosed with type 2 diabetes and a few with type 1 diabetes. So the absolute number of people was low. Indeed very important for precision medicine and the best treatment, and outcome. Many of these patients actually require insulin from start. The researchers found that type 3c diabetes was more common than type 1 diabetes in this group and raise concern that more accurate diagnosis is a must. As for Sweden we have since years the ANDIS-study (6) with very detailed information today of ~15 000 in diabetes newly diagnosed patients, and the prevalence of type 3c diabetes is 1,2%. The British study is interesting and must be replicated. There is probably differences between countries as well.
References:
- http://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_99.2.pdf?sequence=1&isAllowed=y
- https://doi.org/10.1016/S2468-1253(16)30106-6
- http://care.diabetesjournals.org/content/37/Supplement_1/S81?ijkey=56c2101d40bc86f2dde6ee307378f3cc04ca67a3&keytype2=tf_ipsecsha
- https://doi.org/10.2337/db16-1477
- https://doi.org/10.2337/dc17-0542
- http://andis.ludc.med.lu.se/all-new-diabetics-in-scania-andis/
TYPE 1b DIABETES
In a smaller subset of patients, no immune responses or autoantibodies are detected, and the cause of beta cell destruction is unknown. These patients are prone to ketoacidosis, have no evidence for autoimmunity and most are of African or Asian ancestry (1, 2). The heredity is strong and type 1b diabetes is not HLA-associated. Need for insulin replacement come and go in patients.
References:
- http://care.diabetesjournals.org/content/37/Supplement_1/S81?ijkey=56c2101d40bc86f2dde6ee307378f3cc04ca67a3&keytype2=tf_ipsecsha
- https://www.nature.com/articles/nrdp201717


























