What Is Glyset
The main active ingredient of Glyset is miglitol, a unique molecule supplied in pill form of 25, 50, and 100 mg. The substance is a water-soluble pale yellow powder. The inactive ingredients of the drug are:
- starch;
- magnesium stearate;
- microcrystalline cellulose;
- hypromellose;
- titanium dioxide;
- polyethylene glycol;
- polysorbate 80.
The drug is intended for oral use before meals. Miglitol is a substance developed by pharmacists for the treatment of insulin-dependent diabetes mellitus (NIDDM). This molecule is an inhibitor of alpha-glucosidase and a derivative of deoxynogyrimicite. The empirical formula of miglitol looks like this: C8H17NO5.

What Is It Used For
The drug is used as a therapeutic agent for the following pathologies:
- diabetes type 2;
- diabetes mellitus;
- diabetic coma (in DM type II);
- diabetic ketoacidosis (in DM type II).
You should stop using the drug if a patient with diabetes has such pathologies as:
- an inflammatory bowel disease;
- ulcerative colitis;
- Crohn’s disease;
- blockage in your intestines;
- a chronic intestinal disorder that affects your digestion;
- a stomach disorder that causes excess gas;
- diabetic ketoacidosis.
Also, the use of the drug by patients under 18 years without the supervision of a doctor is prohibited due to the poor knowledge of the effects of the molecule on children. As for pregnant patients, according to the results of a number of studies, it can be concluded that the Glyset drug does not affect the fetus.
At the same time, the pharmaceutical product cannot be used by the patient during lactation, since miglitol has been proven to enter the breast milk in a small, but significant amount for the baby.
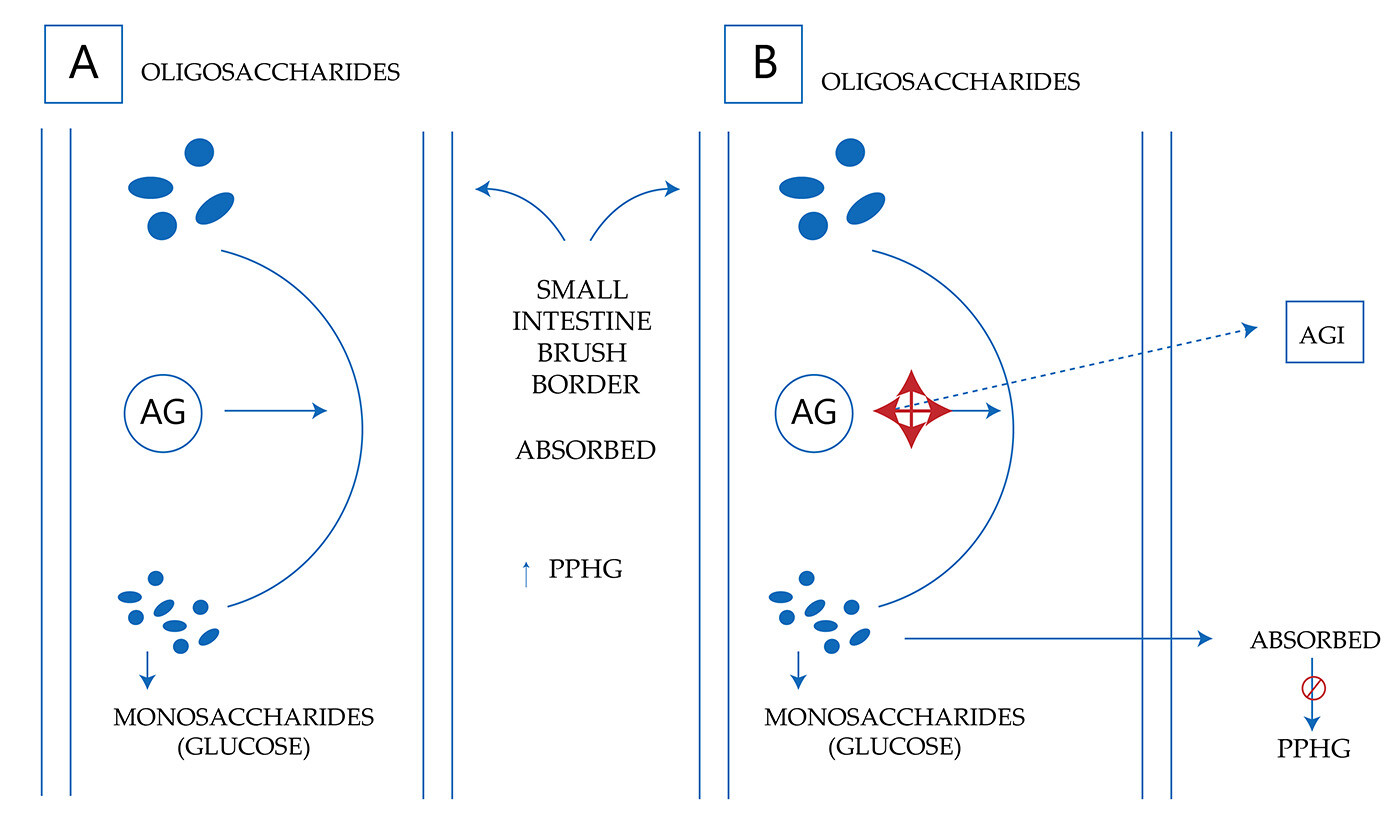
Miglitol Mechanism of Action
Glyset does not enhance insulin secretion. Reversible inhibition of membrane-bound enzymes of intestinal α-glucoside hydrolase causes the antihyperglycemic effect of the miglitol molecule. In the brush border of the small intestine, oligosaccharides and disaccharides are hydrolyzed by membrane-bound intestinal α-glucosidases to glucose and other monosaccharides.
Increased glycemic control with Glyset is manifested in combination therapy with the use of sulfonylurea. It is important that the drug is suitable for patients with lactose intolerance. Miglitol has low inhibitory activity against lactase.
Dosage Forms & Strengths
Therapy of diabetes mellitus with the reception of Glyset is not prescribed as a fixed dosage regimen. Therefore, the dosage is selected individually, taking into account the tolerability of the components of the drug by a particular patient and the expected effectiveness. At the same time, the maximum recommended dose of 100 mg is provided 3 times a day, which cannot be exceeded.
It is recommended to start Glyset treatment with a minimum dose, gradually increasing it. Such a schedule of taking the drug is necessary for two reasons:
- It helps to reduce the negative symptoms from the side of gastrointestinal tract.
- It allows you to determine the minimum allowable dose for glycemic control of the patient.
In practice, the concept of an adequate minimum dose is used – the dosage at which it is possible to reduce glucose levels to normal levels. If you need to identify an adequate minimum dose and determine the therapeutic response to Glyset, plasma glucose can be used as an indicator within 1 hour after a patient’s meal.
The doctor’s task is to identify the minimum dose of the drug necessary to reduce the level of glucose in the blood and glycosylated hemoglobin after taking food for normal or slightly above normal levels. The study can take place both as part of monotherapy and in combination therapy with sulfonylurea.
Initial Dosage
The recommended initial dose of the drug is 25 mg 3 times a day before meals. However, if you need to reduce side effects, a scheme with a similar dosage 1 time a day can be applied with a gradual increase to the recommended frequency of administration. The doctor individually selects the dosage for each patient with diabetes. The regimen, assuming a dose of 25 mg 3 times a day, will reduce the negative effect of the gastrointestinal tract in the first weeks of therapy.
Maintenance Dosage
The standard maintenance dosage of Glyset for diabetic patients is 50 mg 3 times a day with a possible increase in the dose to 100 mg 3 times a day if necessary. Maintenance therapy should replace minimal therapy in 6-8 weeks after the start of taking the drug. This treatment regimen minimizes the likelihood of negative effects from the gastrointestinal tract.
The maintenance dose of Glyset should be used for 3 months. At the end of this period, it is necessary to fix the level of glycosylated hemoglobin in order to find out the therapeutic response. If there are unsatisfactory results, it is recommended to increase the dose to 100 mg 3 times a day.
Maximum Dosage
The maximum allowable dose of Glyset is 100 mg 3 times a day. Despite the fact that an improvement in glycemic control was revealed in a clinical trial with an increase in the dose to 200 mg 3 times a day, an increase in the frequency of gastrointestinal disorders indicates the need to limit the maximum permissible barrier at the level of 100 mg 3 times a day.
It should be mentioned that increasing the dosage above the recommended maximum is likely to provoke side effects associated with the gastrointestinal tract.
Table 1
Results of Monotherapy Study with GLYSET
| HbA1c (%) | 1-hour Postprandial Glucose (mg/dL) | ||||
| Study | Treatment | Mean Change from Baseline *- | Treatment Effect± | Mean Change from Baseline± | Treatment Effect± |
| 1 (U.S.) | Placebo | +0.71 | — | +24 | — |
| GLYSET 50 mg 3 times daily | +0.13 | -0.58++- | -39 | -63++- | |
| 2 (U.S.) | Placebo | +0.47 | — | +15 | — |
| GLYSET 50 mg 3 times daily | -0.22 | -0.69++- | -52 | -67++- | |
| GLYSET 100 mg 3 times daily | -0.28 | -0.75++- | -59 | -74++- | |
| 3 (non-U.S.) | Placebo | +0.18 | — | +2 | — |
| GLYSET 25 mg 3 times daily | -0.08 | -0.26 | -33 | -35++- | |
| GLYSET 50 mg 3 times daily | -0.22 | -0.40 | -45 | -47++- | |
| GLYSET 100 mg 3 times daily | -0.63 | -0.81++- | -62 | -64++- | |
| GLYSET 200 mg 3 times daily §- | -0.84 | -1.02++- | -85 | -87++- | |
| 4 (U.S.) | Placebo | +0.01 | — | +8 | — |
| GLYSET 50 mg 3 times daily | -0.35 | -0.36++- | -20 | -28++- | |
| GLYSET 100 mg 3 times daily | -0.57 | -0.58++- | -25 | -33++- | |
| 5 (U.S.) | Placebo | +0.32 | — | +17 | — |
| GLYSET 100 mg 3 times daily | -0.43 | -0.75++- | -38 | -55++- | |
*- Baseline ranged from 7.54 to 8.72% in these studies
+- The result of subtracking the placebo group average
++- p≤0.05
Common Glyset Side Effects
Glyset has a number of side effects identified as a result of research and post-marketing experience. The following are the main adverse reactions of the body that may occur in patients when taking the Glyset drug.
Gastrointestinal
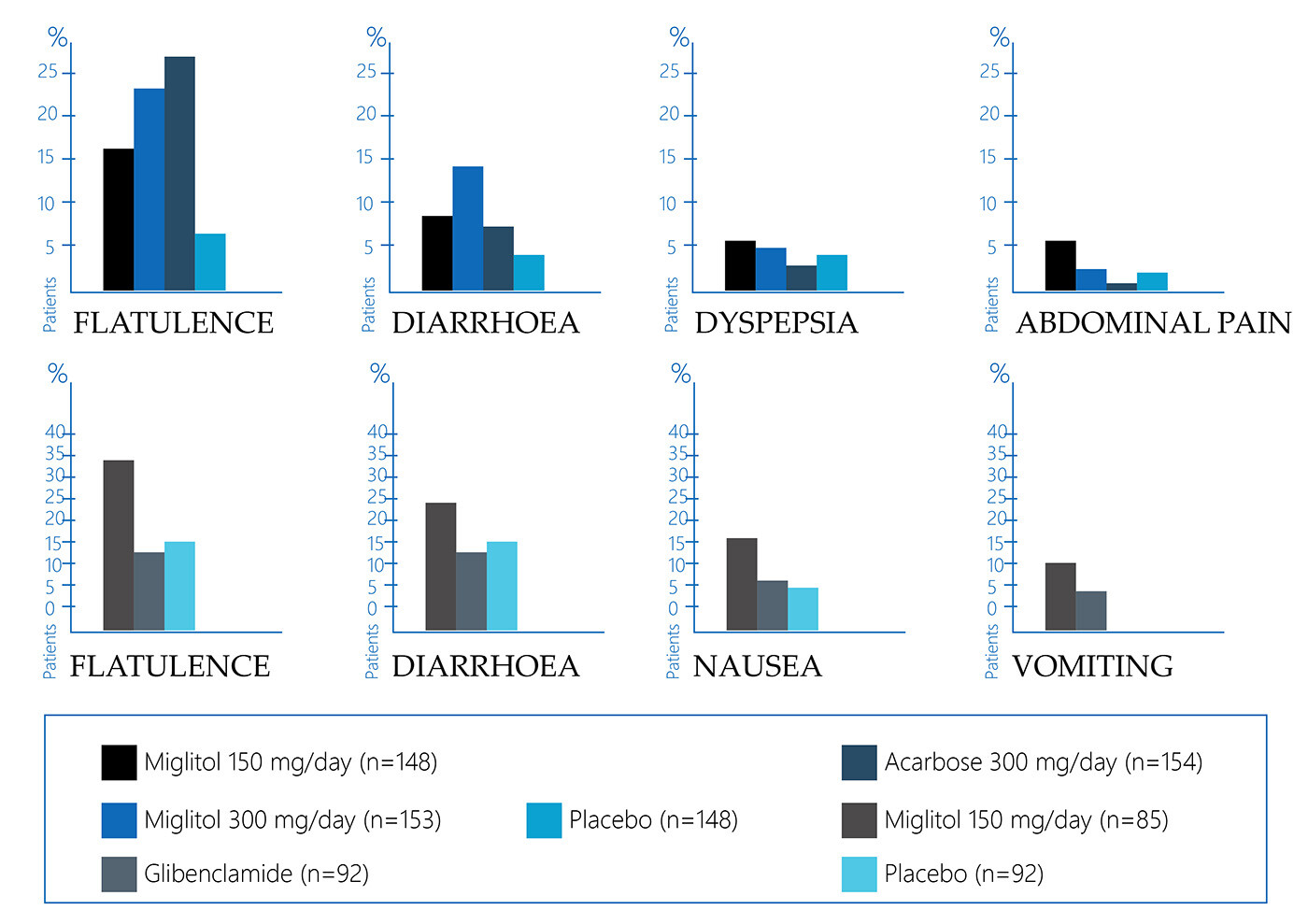
The side effects of the Glyset drug are often associated with a gastrointestinal disorder. A placebo-controlled study revealed that the reception of Glyset may cause such symptoms as:
- abdominal pain – 11.7% of the studied patients;
- diarrhea – 28.7% of the studied patients;
- flatulence – 41.5% of the studied patients.
The study was conducted with a minimum dosage of the drug. It was found that the frequency of side effects in the gastrointestinal tract decreases with the continuation of the course of the drug.
Dermatologic
During the course of a placebo-controlled study, some side effects on the skin were detected in patients taking the minimum dosage of Glyset. A statistically significant skin manifestation was a rash in 4.3% of patients. At the same time, the researchers assessed the rash as not directly related to taking the drug.
Abnormal Laboratory Findings
Patients with diabetes mellitus who took Glyset were more likely to have low serum iron levels (9.2% of such patients were identified). At the same time, this symptom did not persist in most cases. The researchers did not find a link between low serum iron levels and a decrease in hemoglobin, as well as changes in other blood parameters.
Postmarketing Experience
After the Glyset drug was officially approved, some reports of a number of additional negative reactions when taking the drug were recorded. Due to the fact that such messages come from people in a voluntary way, it is not possible to assess the real connection of these symptoms with taking the drug.
This means that the evidentiary potential of such messages is minimal. Nevertheless, it is worth mentioning the following reactions, which are rare, but may occur when taking Glyset:
- intestinal obstruction;
- subileus;
- stomach or bowel pain;
- bloating;
- nausea.
All the side effects reported by patients in the post-marketing experience concerned exclusively the gastrointestinal tract. There were no negative reactions by other body systems to taking Glyset.
Pneumatosis Cystoides Intestinalis
As part of the post-marketing experience, isolated reports of pneumatosis cystoides intestinalis were received from patients who were prescribed Glyset. Researchers associate this side effect with the use of alpha-glucosidase inhibitors, which include Glyset.
This side effect is manifested by a number of symptoms, including:
- constipation;
- rectal bleeding;
- mucus secretion;
- diarrhea.
Also, the described pathology may have complications in the form of intestinal obstruction, intussusception, inversion, or perforation of the intestine. Therefore, patients who encounter symptoms during therapy with Glyset should immediately stop taking the drug and contact a medical specialist for diagnosis as soon as possible.
Glyset Medication Precautions
Despite the fact that Glyset is a certified drug, which is recognized as safe and effective, there are a number of precautions for the use of this pharmaceutical product.
Macrovascular Outcomes
At the moment, there are no clinical studies proving a reduction in macrovascular risk using Glyset. It is important that this thesis is relevant for any drug for diabetes.
General
According to the results of studies and conclusions, Glyset should not provoke hypoglycemia when taken on an empty stomach or after eating, while drugs such as sulfonylureas and insulin can. Therapy using Glyset in combination with sulfonylurea or insulin can cause hypoglycemia. Therefore, a medical specialist needs to adjust the dose of sulfonylurea or insulin as part of combination therapy.
An important point is that Glyset does not delay the absorption of dextrose, so it should be used as a substitute for sucrose. This recommendation is relevant for the treatment of mild or moderate hypoglycemia (in severe cases, intravenous glucose infusion or glucagon injection will be required). Glyset inhibits the hydrolysis of sucrose to fructose and glucose, so sucrose is not suitable for a quick and effective fight against hypoglycemia. Temporary loss of control over blood glucose levels can be triggered by a variety of factors. In this case, insulin therapy is needed. As for kidney failure, the situation is as follows. Since there have been no long-term clinical trials in patients with significant renal impairment, such patients are not recommended to take the drug.
Laboratory Tests
Periodic blood glucose tests allow the doctor and the patient to monitor the therapeutic response of the drug. It is recommended to measure the level of glycosylated hemoglobin for the purpose of long-term glycemic control.
Carcinogenesis, Mutagenesis, and Fertility Problems
The active substance of the Glyset drug called miglitol was administered to mice in doses up to 500 mg/ kg of body weight (5 times the maximum permissible human exposure based on AUC) for 21 months as part of the ongoing study. There was no evidence of carcinogenicity of miglitol. When conducting a test tube with bacterial mutagenesis, it was found that miglitol in vitro is not mutagenic and does not have clastogenic effects in vivo in the mouse micronucleus test.
Hereditary mutations were also not detected in this study. As for studies of the effects on reproductive function, a combined study of the fertility of rats receiving 300 mg/kg of body weight (8 times the maximum permissible exposure to humans) has shown that the drug did not have an adverse effect on reproductive function.
Pregnancy
Glyset is relatively safe for pregnant women, which can be concluded based on animal studies. At the same time, adequate and well-controlled studies have not been conducted with pregnant women by 2022, which restricts doctors from prescribing the drug to pregnant women. As for animal studies, toxicological studies in which rats were given up to 450 mg/ kg of body weight and rabbits up to 200 mg/ kg of body weight of miglitol did not reveal any signs of fetal malformations. The highest doses tested in these studies turned out to be toxic to the mother and fetus (fetal weight loss and delayed ossification of the fetal skeleton).
In general, no detrimental effects on survival, growth, development, behavior, or fertility have been recorded in developmental toxicity studies. However, since Glyset contains miglitol, it should only be used during pregnancy if absolutely necessary.
Nursing Mothers
Miglitol is evidently excreted in small amounts in breast milk (0.02% of the 100 mg dose for the mother) if the mother with diabetes takes a drug containing the molecule. Therefore, it is not recommended to prescribe Glyset as a drug with the active ingredient of miglitol to women during lactation.
Pediatric Use
The safety and efficacy of Glyset in the treatment of children have not been established. The use of Glyset and any other drug containing a similar active substance to treat children should be evaluated in terms of possible risks and potential benefits for the patient.
Geriatric Use
In the USA, a number of studies were conducted in which the goal was to evaluate the effect of the active substance of Glyset on elderly people. Such a study, in which 24% of the subjects were 65+ years old and 3% were 75+ years old, showed that there were no general differences in safety and efficacy among the elderly compared to younger patients. The study of the pharmacokinetics of miglitol in young and elderly men at a dosage of 100 mg 3 times a day for 3 days also revealed no differences between the two groups.
Contraindications
Glyset should not be taken by patients with the following pathologies:
- inflammatory bowel disease;
- ulcers of the large intestine;
- partial intestinal obstruction;
- diabetic ketoacidosis;
- chronic intestinal diseases associated with severe digestive/absorption disorders;
- conditions that may worsen with flatulence.
Hypersensitivity to the components of the drug can also provoke negative effects.
Overdose
Overdose of Glyset pills does not provoke hypoglycemia. A high dose of the drug can cause:
- flatulence;
- diarrhea;
- abdominal discomfort.
Since there are no extra-intestinal reactions to overdose, no systemic symptoms of overdose are expected.
Miglitol Interaction with Other Medications
The task of a number of studies conducted on volunteers was to evaluate the interaction of miglitol and other pharmaceutical products. One of them is the combined intake of miglitol and glyburide. In patients of the control group, the average values of C and AUC were found to be 17% and 25% lower, respectively, when prescribing glyburide with miglitol.
In a parallel study, it was revealed that when visiting the clinic after 6 months and after 1 year by patients taking miglitol and glyburide at the same time, the average C values for glyburide were 16% and 8% lower, respectively, compared with patients taking glyburide alone. There was a tendency to lower AUC and C values for glyburide when co-administered with Glyset, however, the results of the studies do not provide an accurate answer regarding the potential interaction of the drugs.
There have also been studies with metformin. It was revealed that the average AUC and C values in the metformin+miglitol treatment regimen were 12-13% lower than in patients taking miglitol alone. A study of the potential interaction of miglitol and digoxin showed that taking the drug together reduces the average concentrations of digoxin in plasma by 19% and 28%, respectively. At the same time, the indicators were leveled within 14 days.
Studies with ranitidine and propanol have shown that miglitol can significantly reduce the bioavailability of ranitidine and propranolol by 60% and 40%, respectively. The effect of miglitol on the pharmacokinetics or pharmacodynamics of warfarin or nifedipine has not been identified.
Intestinal adsorbents and preparations of digestive enzymes (amylase, pancreatin) can evidently reduce the effect of Glyset. Therefore, the drugs should be taken at different times. At the same time, studies conducted on 12 healthy men showed that simultaneous administration of miglitol with an antacid did not affect the pharmacokinetics of the first.
Drugs Similar to Glyset (Miglitol)
Several drugs akin to Glyset (Miglitol) are available for managing diabetes, particularly focusing on controlling postprandial blood sugar levels.
Acarbose, marketed under the brand name Precose, operates similarly to miglitol by inhibiting alpha-glucosidase enzymes in the intestine, thereby delaying carbohydrate digestion and glucose absorption. Voglibose, another alpha-glucosidase inhibitor, functions comparably to Glyset in delaying carbohydrate absorption from the intestine, aiding in blood sugar regulation post-meals. Both acarbose and voglibose are valuable alternatives for individuals requiring medications that target postprandial hyperglycemia, complementing lifestyle modifications and other antidiabetic therapies in maintaining glycemic control.


























